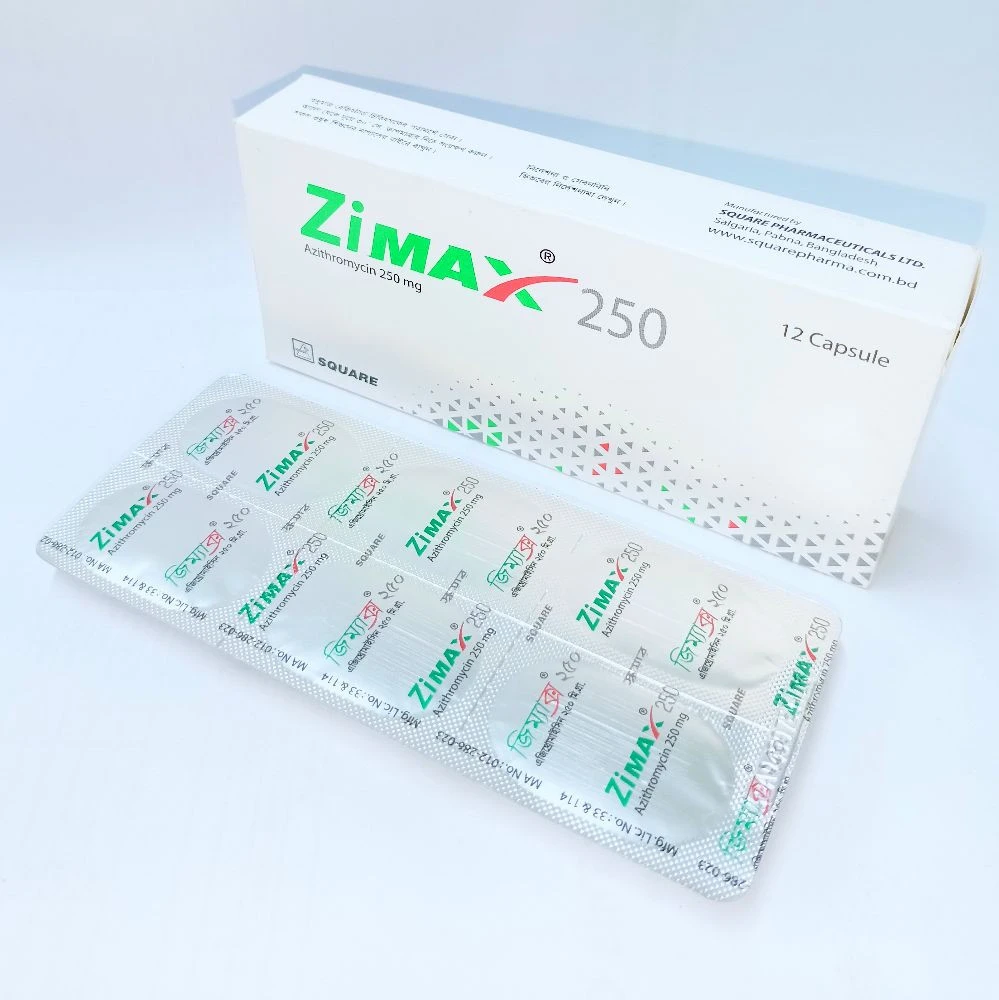
Indications
- Zimax is indicated for infections
(caused by susceptible organisms) in lower respiratory tract infections
including bronchitis and pneumonia, in upper respiratory tract infections
including sinusitis and pharyngitis/tonsillitis, in otitis media, and in skin
and soft tissue infections. In sexually transmitted diseases in men and women,
Zimax is indicated in the treatment of non-gonococcal urethritis and cervicitis
due to Chlamydia trachomatis.
* রেজিস্টার্ড
চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Pharmacology
- Azithromycin is acid-stable and
can therefore be taken orally with no need of protection from gastric acids. It
is readily absorbed; its absorption is greater on an empty stomach. Time to
peak concentration in adults is 2.1 to 3.2 hours for oral dosage forms. Due to
the high concentration in phagocytes, azithromycin is actively transported to
the site of infection. During active phagocytosis, large concentrations of
azithromycin are released. The concentration of azithromycin in the tissues can
be over 50 times higher than in plasma. This is due to ion trapping and the
high lipid solubility.
- Azithromycin's half-life allows a
large single dose to be administered and yet maintain bacteriostatic levels in
the infected tissue for several days. Following a single 500 mg dose, plasma
concentrations of azithromycin declined in a polyphasic pattern with a mean
apparent plasma clearance of 630 mL/min and a terminal elimination half life of
68 hours. The prolonged terminal half-life is thought to be due to extensive
uptake and subsequent release of drug from tissues. Biliary excretion of
azithromycin, predominantly unchanged, is a major route of elimination. Over
the course of a week, approximately 6% of the administered dose appears as
unchanged drug in urine.
- Microbiology: Azithromycin acts
by binding to the 50S ribosomal subunit of susceptible microorganisms and,
thus, interfering with microbial protein synthesis. Nucleic acid synthesis is
not affected. Azithromycin has been shown to be active against most isolates of
the following microorganisms, both in vitro and in clinical infections:
- Aerobic and facultative
gram-positive microorganisms: Staphylococcus aureus, Streptococcus agalactiae,
Streptococcus pneumoniae, Streptococcus pyogenes
- Aerobic and facultative
gram-negative microorganisms: Haemophilus ducreyi, Haemophilus influenzae,
Moraxella catarrhalis, Neisseria gonorrhoeae
- Other microorganisms: Chlamydia
pneumoniae, Chlamydia trachomatis , Mycoplasma pneumoniae , Betalactamase
production should have no effect on azithromycin activity.
- Aerobic and facultative
gram-positive microorganisms: Streptococci (Groups C,F,G), Viridans group
streptococci
- Aerobic and facultative
gram-negative microorganisms: Bordetella pertussis, Legionella pneumophila
- Anaerobic microorganisms:
Peptostreptococcus species, Prevotella bivia
Dosage
Oral-
Adult: 500 mg once daily orally
for 3 days or 500 mg once on day 1, then 250 mg once on days 2-5 for 4 days.
For sexually transmitted diseases caused by Chlamydia trachomatis in adults,
the dose is 1 gm given as a single dose or 500 mg once on day 1, followed by
250 mg once daily for next 2 days may also be given.
Children:
- 10 mg/kg body weight once daily
for 3 days for child over 6 months
- 200 mg (1 teaspoonful) for 3 days
if body weight is 15-25 kg
- 300 mg (1½ teaspoonfuls) for 3
days if body weight is 26-35 kg; 400 mg (2 teaspoonfuls) for 3 days if body
weight is 36-45 kg.
- In typhoid fever, 500 mg (2½
teaspoonfuls) once daily for 7-10 days is given.
- Azithromycin Injection (For IV Infusion
only): The recommended dose of Azithromycin for injection for the treatment of
adult patients with community-acquired pneumonia due to the indicated organisms
is:
- 500 mg as a single daily dose by
the intravenous route for at least two days. Intravenous therapy should be
followed by Azithromycin by the oral route at a single, daily dose of 500 mg,
administered as two 250-mg tablets to complete a 7 to 10-day course of therapy.
The timing of the switch to oral therapy should be done at the discretion of
the physician and in accordance with clinical response.
- The recommended dose of
Azithromycin for the treatment of adult patients with pelvic inflammatory
disease due to the indicated organisms is: 500 mg as a single daily dose by the
intravenous route for one or two days. Intravenous therapy should be followed
by Azithromycin by the oral route at a single, daily dose of 250 mg to complete
a 7-day course of therapy. The timing of the switch to oral therapy should be
done at the discretion of the physician and in accordance with clinical
response. If anaerobic microorganisms are suspected of contributing to the
infection, an antimicrobial agent with anaerobic activity should be
administered in combination with Azithromycin.
- Safety and effectiveness of azithromycin
for injection in children or adolescents under 16 years have not been
established.
* রেজিস্টার্ড
চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Administration
Reconstitution procedure of
suspension-
Step 01: Shake the bottle well to
loosen the powder.
Step 02: Add boiled and cooled
water up to the water mark of the bottle label.
Step 03: Shake until powder is
completely mixed with water.
Azithromycin should be taken at
least 1 hour before or 2 hours after meal.
* রেজিস্টার্ড
চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Interaction
- Antacid: In patients receiving
azithromycin and antacids, azithromycin should be taken at least 1 hour before
or 2 hours after the antacid. Carbamazepine: In a pharmacokinetic interaction
study in healthy volunteers, no significant effect was observed on the plasma
levels of carbamazepine or its active metabolite.
- Cyclosporin: Some of the related
macrolide antibiotics interfere with the metabolism of cyclosporin. In the
absence of conclusive data from pharmacokinetic studies or clinical data
investigating potential interactions between azithromycin and cyclosporine,
caution should be exercised before co-administration of these two drugs. If
coadministrations is necessary, cyclosporin levels should be monitored and the
dose adjusted accordingly.
- Digoxin: Some of the macrolide
antibiotics have been reported to impair the metabolism of digoxin (in the gut)
in some patients. Therefore, in patients receiving concomitant azithromycin and
digoxin the possibility of raised digoxin levels should be borne in mind and
digoxin levels monitored.
- Ergot derivatives: Because of the
theoretical possibility of ergotism, azithromycin and ergot derivatives should
not be co-administered.
- Methylprednisolone: In a
pharmacokinetic interaction study in healthy volunteers, azithromycin had no
significant effect on the pharmacokinetics of methylprednisolone.
- Theophylline: There is no
evidence of any pharmacokinetic interaction when azithromycin and theophylline
are co-administered to healthy volunteers. In general, however, theophylline
levels should be monitored.
- Warfarin: In a pharmacodynamic
interaction study, azithromycin did not alter the anticoagulant effect of a
single 15 mg dose of warfarin administered to healthy volunteers. Zimax and
warfarin may be co-administered, but monitoring of the prothrombin time should
be continued as routinely performed.
- Terfenadine: Zimax did not affect
the pharmacokinetics of terfenadine administered at the recommended dose of 60
mg every 12 hours. Addition of azithromycin did not result in any significant
changes in cardiac repolarisation (QTc interval) measured during the steady
state dosing of terfenadine.
Contraindications
- Azithromycin Dihydrate is
contraindicated in patients hypersensitive to Azithromycin or any other
macrolide antibiotic. Co-administration of ergot derivatives and Azithromycin
is contraindicated. Azithromycin is contraindicated in patients with hepatic
diseases.
Side Effects
- Zimax is well tolerated with a
low incidence of side-effects. Most side-effects observed were mild to moderate
in severity. The majority of side-effects were gastrointestinal in origin with
nauseas, abdominal discomfort (pain/cramps), vomiting, flatulence, diarrhoea
and loose stools being occasionally observed. Allergic reactions such as rash
or photosensitivity have occurred and there have also been rare reports of
serious hypersensitivity reactions. Reversible elevations in liver
transaminases have been seen with a frequency similar to the comparative
macrolides and penicillins used in clinical trials. Rarely, cases of
cholestatic jaundice have been observed. Transient mild reductions in neutrofil
counts have occasionally been observed in clinical trials, although a causal
relationship to azithromycin has not been established. Hearing impairment: In
investigational studies where higher doses were used for prolonged periods of
time, reversible hearing impairment was seen in some patients.
Pregnancy & Lactation
- Pregnancy Category of
Azithromycin Dihydrate is B. Animal reproduction studies have demonstrated that
Azithromycin has no evidence of harm to the fetus. There are no adequate and
well controlled studies in pregnant women. Since animal reproduction studies
are not always predictive of human response, Azithromycin should be used during
pregnancy only if adequate alternatives are not available. It is not known
whether Azithromycin is secreted in breast milk. So, caution should be
exercised when Azithromycin is administered to nursing women.
Precautions & Warnings
- As with erythromycin and other
macrolides, rare serious allergic reactions, including angioneurotic oedema and
anaphylaxis, has been reported. Some of these reactions with azithromycin have
resulted in recurrent symptoms and required a long period of observation and
treatment.
Use in Special Populations
- Use in renal impairment: No dose
adjustment is needed in patients with mild renal impairment (creatinine
clearance >40 ml/min), but there are no data regarding azithromycin in
patients with more severe renal impairment, thus caution should be exercised in
using azithromycin in these patients.
- Use in hepatic impairment: As the
liver is the principal route of excretion of azithromycin, it should not be
used in patients with hepatic disease.
- Effects on ability to drive and
use machines: There is no evidence to suggest that azithromycin may have an
effect on a patient’s ability to drive or operate machinery.
Overdose Effects
- There is no data on overdosage
with Zimax. Typical symptoms of overdosage with macrolide antibiotics include
hearing loss, severe nausea, vomiting and diarrhoea. Gastric lavage and general
supportive measures are indicated.
Storage Conditions
- Keep in a dry place away from
light and heat. Keep out of the reach of children.