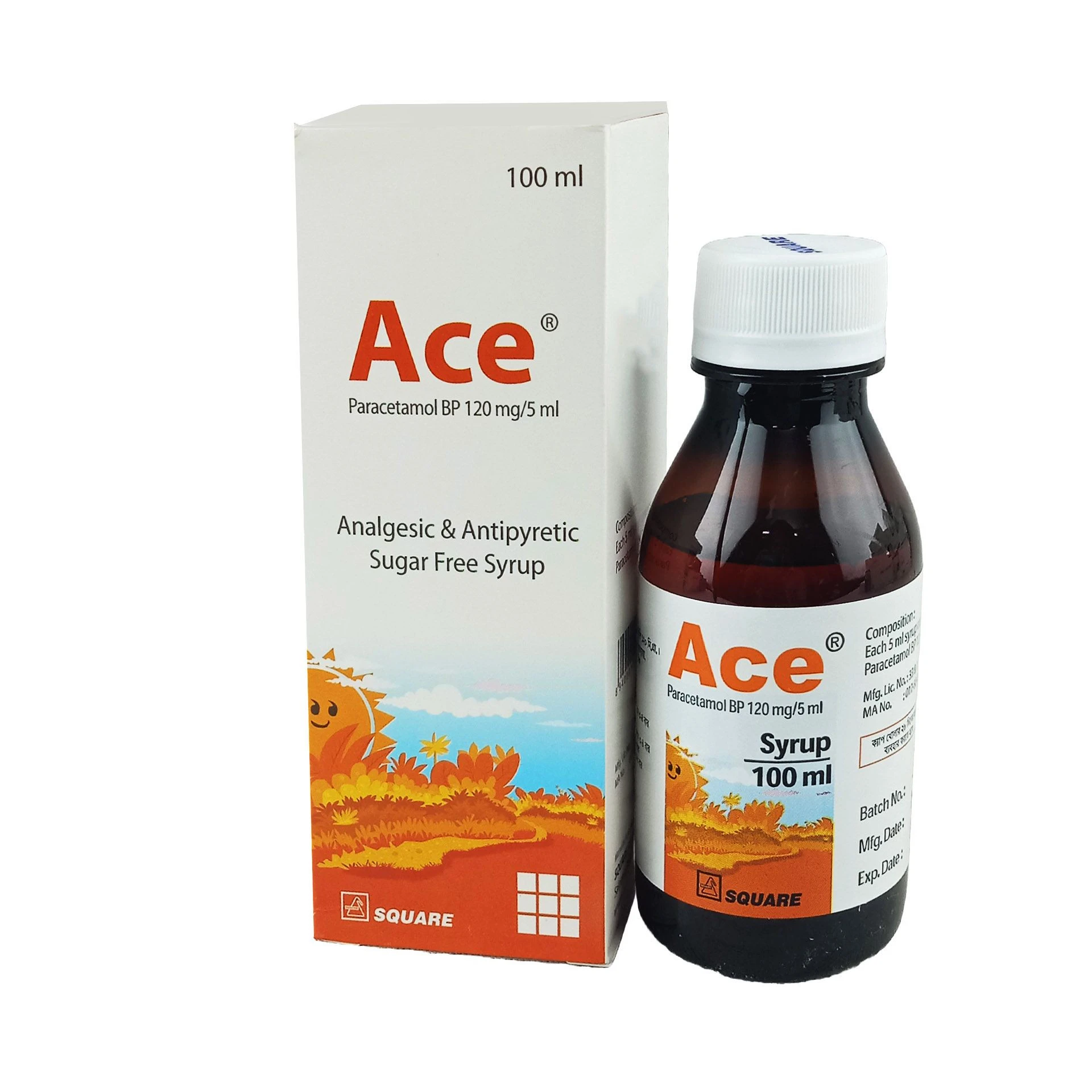
Indications
This is indicated as an adjunct
to diet and exercise to improve glycemic control in adults with type 2 diabetes
mellitus when treatment with both sitagliptin and metformin is appropriate.
Important limitations of use:
This should not be used in
patients with type 1 diabetes or for the treatment of diabetic ketoacidosis, as
it would not be efective in these settings.
* রেজিস্টার্ড
চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Pharmacology
This tablet combines two antihyperglycemic
agents with complementary mechanisms of action to improve glycemic control in
patients with type 2 diabetes. Sitagliptin, a dipeptidyl peptidase-4 (DPP-4)
inhibitor, and Metformin HCl, a member of the biguanide class. Sitagliptin is a
dipeptidyl peptidase-4 (DPP-4) inhibitor, which is believed to exert its
actions in patients with type 2 diabetes by slowing the inactivation of
incretin hormones. Incretin hormones, including glucagon-like peptide-1 (GLP-1)
and glucose-dependent insulinotropic polypeptide (GIP), are released by the
intestine throughout the day, and levels are increased in response to a meal.
These hormones are rapidly inactivated by the enzyme, DPP-4. The incretins are
part of an endogenous system involved in the physiologic regulation of glucose
homeostasis. When blood glucose concentrations are normal or elevated then
GLP-1 and GIP increase insulin synthesis and release from pancreatic beta cells
by intracellular signaling pathways involving cyclic AMP. GLP-1 also lowers glucagon
secretion from pancreatic alpha cells, leading to reduced hepatic glucose
production. By increasing and prolonging active incretin levels, Sitagliptin
increases insulin release and decreases glucagon levels in the circulation in a
glucose-dependent manner. The pharmacologic mechanism of action of Metformin
HCl is different from other classes of oral antihyperglycemic agents. Metformin
HCl decreases hepatic glucose production, decreases intestinal absorption of
glucose and increases peripheral glucose uptake and utilization.
Dosage & Administration
Dose of film-coated tablet: The
dosage of this tablet should be individualized on the basis of the patient's
current regimen, efectiveness, and tolerability while not exceeding the maximum
recommended daily dose of 100 mg sitagliptin and 2000 mg metformin. Initial
combination therapy or maintenance of combination therapy should be
individualized and left to the discretion of the health care provider.
This tablet should generally be
given twice daily with meals, with gradual dose escalation, to reduce the
gastrointestinal (GI) side efects due to metformin.
The starting dose of this tablet
should be based on the patient’s current regimen. This tablet should be given
twice daily with meals.
The recommended starting dose in
patients not currently treated with metformin is 50 mg sitagliptin/500 mg
metformin hydrochloride twice daily, with gradual dose escalation recommended
to reduce gastrointestinal side efects associated with metformin.
The starting dose in patients
already treated with metformin should provide sitagliptin dosed as 50 mg twice
daily (100 mg total daily dose) and the dose of metformin already being taken.
For patients taking metformin 850 mg twice daily, the recommended starting dose
of this tablet is 50 mg sitagliptin/1000 mg metformin hydrochloride twice
daily.
No studies have been performed
specifcally examining the safety and efcacy of Sitagliptin Phosphate
Monohydrate INN/Metformin Hydrochloride BP in patients previously treated with
other oral antihyperglycemic agents and switched to Sitagliptin Phosphate
Monohydrate INN/Metformin Hydrochloride BP. Any change in therapy of type 2
diabetes should be undertaken with care and appropriate monitoring as changes
in glycemic control can occur.
Dose of extended-release tablet:
Administer once daily with a meal preferably in the evening. Gradually escalate
the dose to reduce the gastrointestinal side effects due to Metformin. May
adjust the dosing based on effectiveness and tolerability while not exceeding
the maximum recommended daily dose of 100 mg Sitagliptin and 2000 mg Metformin
extended-release. Maintain the same total daily dose of Sitagliptin and
Metformin when changing between film-coated tablet and extended-release tablet,
without exceeding the maximum recommended daily dose of 2000 mg Metformin
extended-release.
Patients using two
extended-release tablets (such as two 50/500 or two 50/1000 tablets) should
take the two tablets together once daily. The 100 mg Sitagliptin/1000 mg
Metformin HCI extended-release tablet should be taken as a single tablet once
daily.
Patients treated with an insulin
secretagogue or insulin: Co-administration of the combination with an insulin
secretagogue (e.g., sulfonylurea) or insulin may require lower doses of the
insulin secretagogue or insulin to reduce the risk of hypoglycemia.
* রেজিস্টার্ড
চিকিৎসকের পরামর্শ মোতাবেক ঔষধ সেবন করুন'
Interaction
Cationic Drugs: Cationic drugs
eliminated by renal tubular secretion: Use with caution.
Phenprocoumon: Metformin may
decrease the anticoagulant effect of phenprocoumon. Therefore, close monitoring
of the INR is recommended.
Levothyroxine: Levothyroxine can
reduce the hypoglycemic effect of metformin. Monitoring of blood glucose levels
is recommended, especially when thyroid hormone therapy is initiated or
stopped, and the dosage of metformin must be adjusted if necessary.
Contraindications
This tablet is contraindicated in
patients with:
Renal disease or renal
dysfunction, e.g., as suggested by serum creatinine levels ≥1.5 mg/dL [males],
≥1.4 mg/dL [females] or abnormal creatinine clearance which may also result
from conditions such as cardiovascular collapse (shock), acute myocardial
infarction, and septicemia
Acute or chronic metabolic
acidosis, including diabetic ketoacidosis, with or without coma.
History of a serious
hypersensitivity reaction to this tablet or sitagliptin, such as anaphylaxis or
angioedema.
This tablet should be temporarily
discontinued in patients undergoing radiologic studies involving intravascular
administration of iodinated contrast materials, because use of such products
may result in acute alteration of renal function.
Side Effects
The most common adverse reactions
reported in ≥5% of patients simultaneously started on sitagliptin and metformin
and more commonly than in patients treated with placebo were diarrhea, upper
respiratory tract infection, and headache.
Adverse reactions reported in ≥5%
of patients treated with sitagliptin in combination with sulfonylurea and
metformin and more commonly than in patients treated with placebo in
combination with sulfonylurea and metformin were hypoglycemia and headache.
Hypoglycemia was the only adverse
reaction reported in ≥5% of patients treated with sitagliptin in combination
with insulin and metformin and more commonly than in patients treated with
placebo in combination with insulin and metformin.
Nasopharyngitis was the only
adverse reaction reported in ≥5% of patients treated with sitagliptin
monotherapy and more commonly than in patients given placebo.
The most common (>5%) adverse
reactions due to initiation of metformin therapy are diarrhea, nausea/vomiting,
fatulence, abdominal discomfort, indigestion, asthenia, and headache.
Pregnancy & Lactation
Pregnancy Category B. There are
no adequate and well-controlled studies in pregnant women with Sitagliptin
Phosphate Monohydrate INN/Metformin Hydrochloride BP or its individual
components; therefore, the safety of Sitagliptin Phosphate Monohydrate
INN/Metformin Hydrochloride BP in pregnant women is not known. This tablet
should be used during pregnancy only if clearly needed.
It is not known whether
sitagliptin is excreted in human milk. Because many drugs are excreted in human
milk, caution should be exercised when this tablet is administered to a nursing
woman.
Precautions & Warnings
Lactic Acidosis-
Lactic acidosis can occur due to
metformin accumulation. The risk increases with conditions such as sepsis,
dehydration, excess alcohol intake, hepatic insufciency, renal impairment, and
acute congestive heart failure.
Symptoms include malaise,
myalgias, respiratory distress, increasing somnolence, and nonspecifc abdominal
distress. Laboratory abnormalities include low pH, increased anion gap and
elevated blood lactate.
If acidosis is suspected,
discontinue this tablet and hospitalize the patient immediately.
Regular monitoring of
thyroid-stimulating hormone (TSH) levels is recommended in patients with
hypothyroidism.
Long-term treatment with
metformin has been associated with a decrease in vitamin B12 serum levels which
may cause peripheral neuropathy. Monitoring of the vitamin B12 level is
recommended.
Others-
Do not use this tablet in
patients with hepatic disease.
There have been postmarketing
reports of acute renal failure, sometimes requiring dialysis. Before initiating
this tablet and at least annually thereafter, assess renal function and verify
as normal.
There have been postmarketing
reports of acute pancreatitis, including fatal and non-fatal hemorrhagic or
necrotizing pancreatitis. If pancreatitis is suspected, promptly discontinue
this tablet.
Measure hematologic parameters
annually.
Warn patients against excessive
alcohol intake.
May need to discontinue this
tablet and temporarily use insulin during periods of stress and decreased
intake of fluids and food as may occur with fever, trauma, infection or
surgery.
Promptly evaluate patients
previously controlled on this tablet who develop laboratory abnormalities or
clinical illness for evidence of ketoacidosis or lactic acidosis.
When used with an insulin
secretagogue (e.g., sulfonylurea) or with insulin, a lower dose of the insulin
secretagogue or insulin may be required to reduce the risk of hypoglycemia.
There have been postmarketing
reports of serious allergic and hypersensitivity reactions in patients treated
with sitagliptin (one of the components of this tablet ), such as anaphylaxis,
angioedema, and exfoliative skin conditions including Stevens-Johnson syndrome.
In such cases, promptly stop this tablet, assess for other potential causes,
and institute appropriate monitoring and treatment, and initiate alternative
treatment for diabetes.
There have been no clinical
studies establishing conclusive evidence of macrovascular risk reduction with
Sitagliptin Phosphate Monohydrate INN/Metformin Hydrochloride BP or any other
anti-diabetic drug.
Overdose Effects
Sitagliptin: In the event of an
overdose, it is reasonable to employ the usual supportive measures, e.g.,
remove unabsorbed material from the gastrointestinal tract, employ clinical
monitoring (including obtaining an electrocardiogram), and institute supportive
therapy as indicated by the patient's clinical status. Sitagliptin is modestly
dialyzable. Prolonged hemodialysis may be considered if clinically appropriate.
It is not known if sitagliptin is dialyzable by peritoneal dialysis.
Metformin hydrochloride: Overdose
of metformin hydrochloride has occurred, including ingestion of amounts greater
than 50 grams. Metformin is dialyzable with a clearance of up to 170 mL/min under
good hemodynamic conditions. Therefore, hemodialysis may be useful for removal
of accumulated drug from patients in whom metformin overdosage is suspected.
Pancreatitis may occur in the context of a metformin overdose.
Therapeutic Class
Combination Oral hypoglycemic
preparations
Storage Conditions
Store below 25°C in a dry place
away from light. Keep the medicines in a safe place, out of the reach of
children. Do not use later than the date of expiry. To be dispensed only on the
prescription of a registered physician.
No review given yet!
 Fast Delivery all across the country
Fast Delivery all across the country
 Safe Payment
Safe Payment
 7 Days Return Policy
7 Days Return Policy
 100% Authentic Products
100% Authentic Products





You need to Sign in to view this feature
This address will be removed from this list