CAUTION
Caution is advised when consuming
alcohol with Febus 80. Please consult your doctor.
CONSULT YOUR DOCTOR
Febus 80 may be unsafe to use
during pregnancy. Although there are limited studies in humans, animal studies
have shown harmful effects on the developing baby. Your doctor will weigh the
benefits and any potential risks before prescribing it to you. Please consult
your doctor.
CONSULT YOUR DOCTOR
Febus 80 is probably unsafe to
use during breastfeeding. Limited human data suggests that the drug may pass
into the breastmilk and harm the baby.
UNSAFE
Febus 80 may cause side effects
which could affect your ability to drive. Febus 80 may cause dizziness,
sleepiness, blurred vision, numbness or tingling sensation. This may affect
your driving ability.
CAUTION
Febus 80 should be used with
caution in patients with severe kidney disease. Dose adjustment of Febus 80 may
be needed. Please consult your doctor. Limited information is available
regarding the use of Febus 80 in these patients.
CAUTION
Febus 80 should be used with
caution in patients with severe liver disease. Dose adjustment of Febus 80 may
be needed. Please consult your doctor. Limited information is available
regarding the use of Febus 80 in these patients.
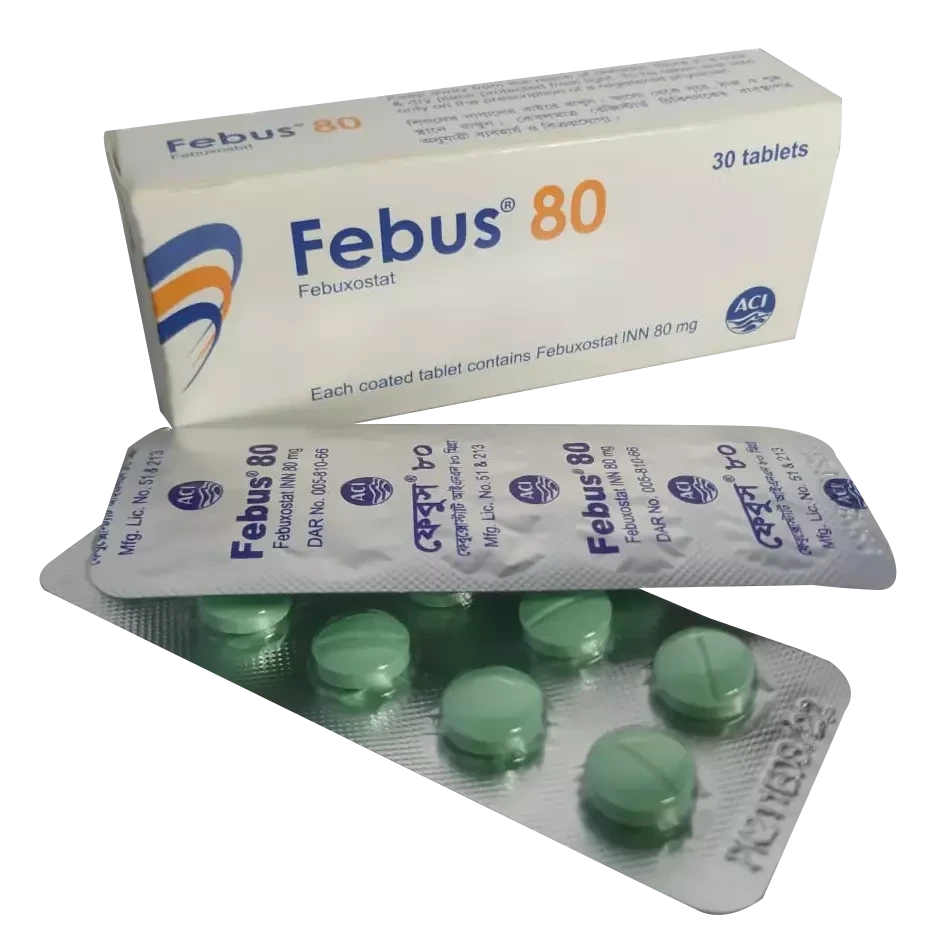
Medicine Overview of Febus
80mg Tablet
Introduction
Febus 80 is a medicine used to
treat and prevent gout. Gout happens when there is too much uric acid in your
body and it forms into crystals which can appear around your joints leading to
painful and swollen joints. This medicine helps to keep uric acid levels low.
Febus 80 can be taken with or without food. You should keep taking it as
recommended by your doctor even when you are not having a gout attack. If you
stop, your symptoms may get worse because of the formation of more crystals in
your joints. You can help yourself by making some changes to your diet (eg.
avoiding alcohol and non-vegetarian foods) and drinking plenty of fluids. Some
of the common side effects of this medicine are headache, diarrhea, nausea,
skin rash and a temporary increase in gout symptoms (rapid onset of severe
pain, warmth, and redness in the joint). However, do not stop taking it. Your
doctor may advise you some painkillers and additional medicines to help you
reduce or prevent these symptoms. Rarely, some people may get a severe allergic
reaction (difficulty in breathing, hives, swelling of face and throat,
weakness) which may need urgent medical attention. Also, tell your doctor right
away if you get any symptoms of liver disease like persistent nausea, dark
urine or yellowing of the eyes or skin. Before taking this medicine, let your
doctor know if you have any heart problems, stroke, thyroid problems, or kidney
or liver problems. If you are pregnant or breastfeeding, this medicine is best
avoided. Check with your doctor. You will need regular blood tests while taking
this medicine to check that your liver is working properly.
Uses of Febus 80
Gout
Side effects of Febus 80
Common
Diarrhea
Headache
Increased liver enzymes
Nausea
Skin rash
Gout flares
How to use Febus 80
Take this medicine in the dose
and duration as advised by your doctor. Swallow it as a whole. Do not chew,
crush or break it. Febus 80 may be taken with or without food, but it is better
to take it at a fixed time.
How Febus 80 works
Febus 80 is a xanthine oxidase
inhibitor. It works by decreasing blood uric acid, which is the chemical that
causes gout.
What if you forget to take Febus
80?
If you miss a dose of Febus 80,
take it as soon as possible. However, if it is almost time for your next dose,
skip the missed dose and go back to your regular schedule. Do not double the
dose.
Quick Tips
Your doctor has prescribed Febus
80 to reduce episodes of gout attack.
Take it at the same time every
day, with or without food.
Take plenty of fluids (2-3
litres) daily while on Febus 80.
When you first start taking this
medicine, you might have more gout attacks. Do not stop Febus 80 on having an
acute attack of gout as that could make an attack worse.
Do not consume alcohol while
taking this medicine as it may cause your gout to flare up.
Stop taking this medicine and
inform your doctor straight away if you have symptoms like rash, itchiness,
difficulties in breathing, fever or swelling of limbs or face.
Brief Description
Indication
Gout, Hyperuricemia
Administration
May be taken with or without
food. May be taken w/o regard to antacid use.
Adult Dose
Chronic Gout 40 mg/day PO
initially; maintenance: 40-80 mg/day; increased if serum uric acid is >6
mg/mL after 2 weeks Hepatic impairment Mild to moderate (Child-Pugh class A or
B): Dosage adjustment not necessary Severe (Child-Pugh class C): Data not
available; use with caution
Child Dose
Safety and efficacy not
established
Renal Dose
Renal impairment Mild to moderate
(CrCl 30-89 mL/min): Dosage adjustment not necessary Severe (CrCl <30
mL/min): Data not available; use with caution
Contraindication
Patients being treated with
azathioprine, mercaptopurine, or theophylline.
Mode of Action
Febuxostat selectively inhibits
xanthine oxidase, the enzyme that catalyses the conversion of hypoxanthine to
xanthine to uric acid, thereby decreasing serum concentrations of uric acid.
Precaution
Cardiovascular death Patients
with established cardiovascular (CV) disease treated with febuxostat had a
higher rate of CV death compared with those treated with allopurinol in a CV
outcomes study. Evaluate risks and benefits when prescribing febuxostat or
continuing treatment. Gout Flare: An increase in gout flares is frequently
observed during initiation of anti-hyperuricemic agents, including Febustat. If
a gout flare occurs during treatment, Febustat need not be discontinued.
Prophylactic therapy (i.e., non-steroidal anti-inflammatory drug (NSAID) or
colchicine upon initiation of treatment) may be beneficial for up to six
months. Lactation: Unknown whether drug is excreted into breast milk; use with
caution
Side Effect
>1% Arthralgia,Elevated liver
function test (LFT) results,Liver function abnormalities,Nausea,Rash
Pregnancy Category Note
Pregnancy Limited available data
in pregnant women are insufficient to inform a drug associated risk of adverse
developmental outcomes Animal data No adverse developmental effects observed in
embryo-fetal development studies with oral administration of febuxostat to
pregnant rats and rabbits during organogenesis at doses that produced maternal
exposures up to 40 and 51 times, respectively, the exposure at the maximum
recommended human dose (MRHD) No adverse developmental effects observed in a
pre- and postnatal development study with administration of febuxostat to
pregnant rats from organogenesis through lactation at an exposure ~11 times the
MRHD Lactation There are no data on presence of febuxostat in human milk,
effects on breastfed infant, or on milk production; drug is present in rat milk
Consider the developmental and health benefits of breastfeeding along with
mother’s clinical need for therapy and any potential adverse effects on
breastfed child from therapy or from underlying maternal condition
Interaction
May increase plasma conc of
mercaptopurine, azathioprine. Alteration of metabolism of xanthine oxidase
substrate drugs (eg theophylline).